Researchers have unveiled a new form of ice, designated as ice XXI, which forms at ambient temperatures under high pressure. This discovery was made by scientists at the Korea Research Institute of Standards and Science (KRISS), highlighting the complexities of water and its diverse structural forms. Unlike traditional ice, this new variant demonstrates unique characteristics that could reshape our understanding of ice formation in extreme conditions, both on Earth and beyond.
For over a century, scientists have studied the various types of ice that water can form, with more than 20 distinct crystalline structures identified so far. These forms range from familiar ice, such as that found in household freezers, to exotic varieties that exist under extreme pressures seen in the Earth’s mantle or on distant celestial bodies. The recent work by KRISS scientists adds a new dimension to this field of research.
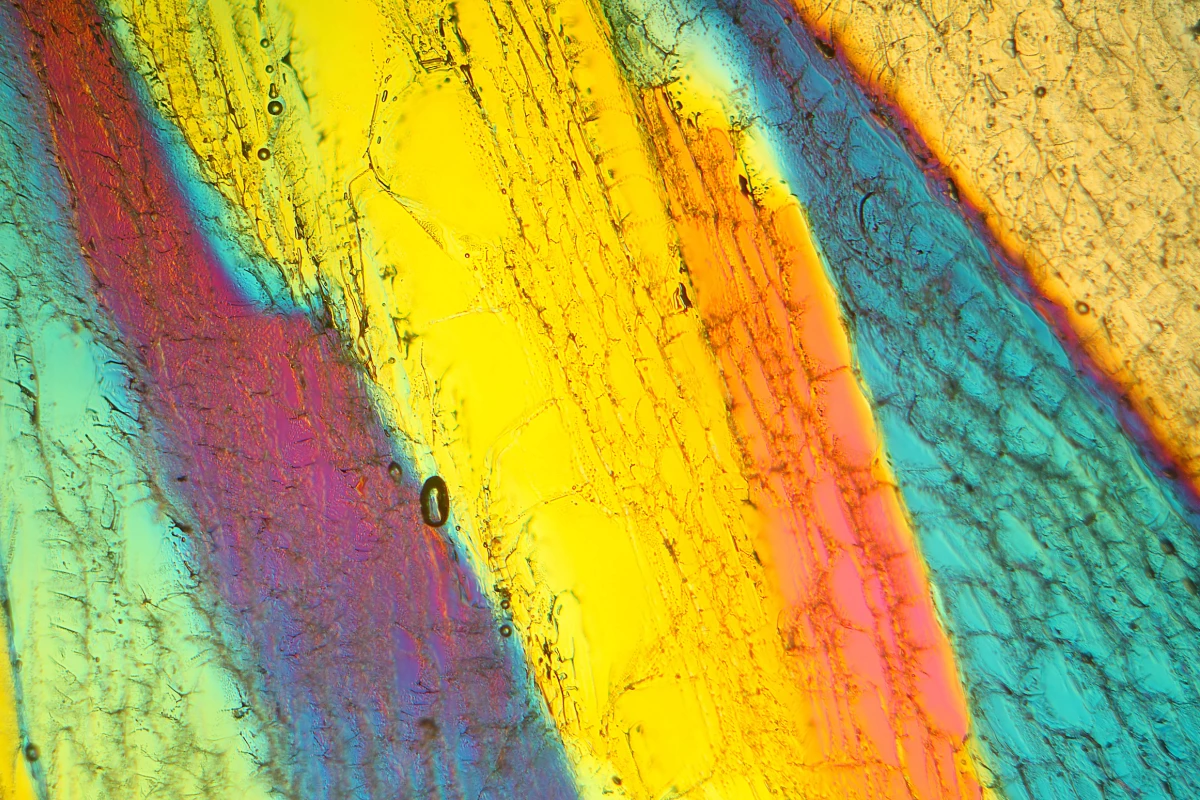
In their experiments, the team utilized advanced techniques involving diamond anvil cells and X-ray lasers to examine how super-compressed water behaves at room temperature. Instead of freezing uniformly, the water exhibited multiple freeze–melt cycles within the pressure range where ice VI typically forms. It was during these transformations that the researchers identified ice XXI, marking a significant breakthrough in the study of water’s properties.
Understanding Ice XXI’s Unique Structure
What sets ice XXI apart is its distinct atomic arrangement, which is unlike any of the previously known ice types. It exists in a metastable state, indicating that while it is not the most stable form, it can persist for a significant duration under the right conditions. As Geun Woo Lee, a scientist at KRISS, explained, “Rapid compression of water allows it to remain liquid at higher pressures, where it should have crystallized into ice VI.”
The team conducted their experiments by loading ultra-pure water into a thin metal chamber. They then employed high-speed cameras and laser-based sensors to monitor the freezing and melting processes in real-time. This innovative approach allowed them to capture detailed snapshots of the transitions, providing insights into the structural changes occurring within the water molecules.
To pinpoint the exact moment of transformation into ice XXI, researchers utilized powerful X-ray beams from a synchrotron. The scattered X-ray signals were meticulously analyzed using a specialized program, aiding in the identification of ice formation pathways. They discovered that water does not freeze in a single step; rather, it follows at least five different routes, showcasing its complex behavior under pressure.
Implications for Future Research
The implications of this discovery extend beyond mere academic curiosity. The findings suggest that additional high-temperature metastable ice phases may exist, which could offer new avenues for understanding the composition of icy moons and planets. As Rachel Husband, another member of the research team, noted, “The findings suggest that a greater number of high-temperature metastable ice phases and their associated transition pathways may exist, potentially offering new insights into the composition of icy moons.”
The research has been published in the esteemed journal Nature Materials, contributing to the ongoing dialogue about water’s behavior in various environments. By uncovering the nuances of ice XXI, scientists hope to deepen our understanding of icy worlds, including those that harbor potential extraterrestrial life.
This groundbreaking work not only enriches our knowledge of ice but also opens up new discussions about the conditions necessary for life in extreme environments, both on Earth and across the universe. As researchers continue to explore the complexities of water, the implications of their findings could have far-reaching consequences for multiple fields, including planetary science, geology, and astrobiology.