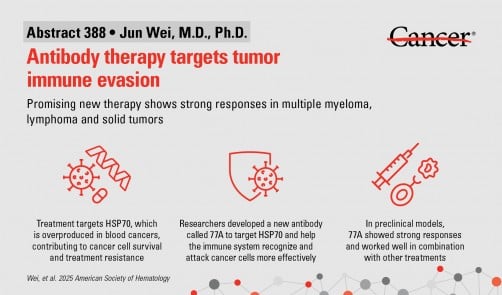
Researchers at The University of Texas MD Anderson Cancer Center have developed a promising new antibody therapy, designated 77A, which enhances the immune response against various blood cancers and solid tumors. This innovative treatment was presented by Jun Wei, M.D., Ph.D., during the 67th American Society of Hematology (ASH) Annual Meeting held on December 6, 2025. The study indicates that 77A could potentially overcome treatment resistance in cancers such as myeloma and lymphoma.
Mechanism of Action and Laboratory Results
The 77A antibody functions by targeting HSP70, a heat shock protein that is often overproduced in certain cancers. This protein assists tumors in evading immune detection, thereby creating an environment that suppresses immune responses and promotes cancer cell survival. By activating T cells and natural killer (NK) cells, 77A effectively reshapes the tumor microenvironment, leading to enhanced immune activity against cancer cells.
In laboratory models, 77A demonstrated significant antitumor effects. It not only improved the efficacy of immune cells in detecting and destroying cancer cells but also worked synergistically with existing treatments, including chemotherapy, radiation therapy, and immune checkpoint blockade. The antibody’s potential extends to novel therapies such as adoptive T cell therapy, where patients receive lab-grown immune cells tailored to combat their cancer.
Future Directions and Clinical Trials
Initial tests using human immune cells indicated that 77A could enhance immune responses in healthy donors, suggesting a promising pathway for clinical applications. The research team, led by principal investigator Robert Z. Orlowski, M.D., Ph.D., expressed optimism regarding the therapy’s versatility. “These results give us confidence that 77A could become a versatile immunotherapy,” Orlowski stated, emphasizing the need to advance a humanized version of the antibody into clinical trials.
The development of the 77A humanized antibody is underway, with plans to initiate clinical trials aimed at evaluating its effectiveness across multiple cancer types. The study received support from Blood Cancer United, formerly known as the Leukemia & Lymphoma Society, highlighting the collaborative efforts in advancing cancer treatment research.
As the scientific community eagerly anticipates the outcomes of future trials, the findings from MD Anderson provide a beacon of hope for patients facing the challenges of treatment-resistant cancers.