Researchers at the University of Minnesota have developed a groundbreaking one-pot method for synthesizing aryne precursors from readily available carboxylic acids. This innovative approach allows for the generation of these highly reactive organic intermediates using either blue light or mild heat, significantly simplifying the process of creating complex aromatic molecules.
The findings, published in the journal Nature on November 11, 2025, mark a significant advancement in synthetic chemistry, particularly in drug discovery and agricultural chemistry. Arynes, characterized by a triple bond within an aromatic ring, are valuable due to their ability to form bonds with various functional groups. Despite their potential, traditional methods for producing arynes have been complex and often unsuitable for sensitive compounds.
Overcoming Synthesis Challenges
Building aromatic compounds with multiple substitutions presents a core challenge in synthetic chemistry. Arynes, with their highly strained structure and dual reactive ends, can facilitate a range of chemical transformations. Yet, existing methods for creating arynes have been limited. Traditional techniques require harsh bases to remove protons, followed by halide elimination, making them less effective for sensitive molecules.
Moreover, previous attempts to use thermally activated precursors have resulted in highly explosive reactions, while ultraviolet light methods often produced more unwanted reactions than desired outcomes. This prompted the research team to explore a simpler, milder method for generating diverse aryne derivatives.
Innovative One-Pot Synthesis
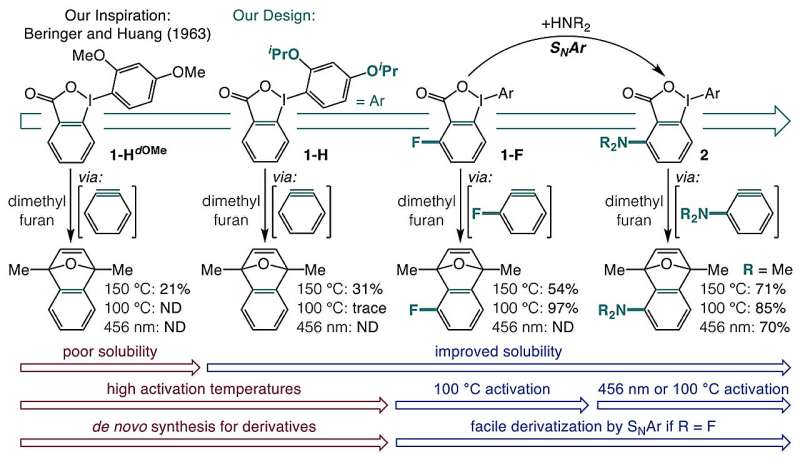
The research team hypothesized that the solution could lie in the use of o-iodoniobenzoates, which they sought to transform into aryne precursors. By developing a one-pot synthesis of these precursors from carboxylic acids, the team aimed to create a method that could be activated with visible light or mild heat.
Initially, o-iodoniobenzoates posed challenges due to their poor solubility and tendency for unwanted side reactions. Through careful experimentation, the researchers discovered that introducing isopropoxy groups enhanced solubility and reduced side reactions. This innovation paved the way for producing aryne precursors that could be activated by blue light or heating to 100 °C.
Further investigation revealed that the heat activation process is influenced by a field effect created by chemical groups adjacent to the carboxylate on the aromatic ring. This electronic field promotes decarboxylation, while blue light at 398 nm excites the molecule to a triplet state, leading to the formation of arynes by breaking the bond with iodine and releasing carbon dioxide.
The researchers emphasize that this new one-pot approach significantly expands access to a variety of aryne precursors starting from common carboxylic acids. It is compatible with numerous functional groups and simplifies the synthesis of complex aromatic compounds, thereby opening doors to previously unexplored chemical opportunities.
The study, led by Chris M. Seong and his team, highlights the potential for this method to enhance the efficiency of synthesizing crucial compounds in pharmaceuticals and agrochemicals. The implications of this research reach far beyond the laboratory, potentially impacting various industries reliant on advanced chemical synthesis.
This article was authored by Sanjukta Mondal, edited by Lisa Lock, and fact-checked by Robert Egan. The work reflects a commitment to independent science journalism, emphasizing the importance of credible reporting in the field.
For more information, see the study by Chris M. Seong et al in Nature, DOI: 10.1038/s41586-025-09830-1.