Research has unveiled the critical role of a mechanosensor in the rapid response of the Venus flytrap (Dionaea muscipula) to prey. A study led by Hiraku Suda and colleagues, published in Nature Communications, identifies the mechanosensor known as DmMSL10 as essential for the plant’s ability to detect and capture insects.
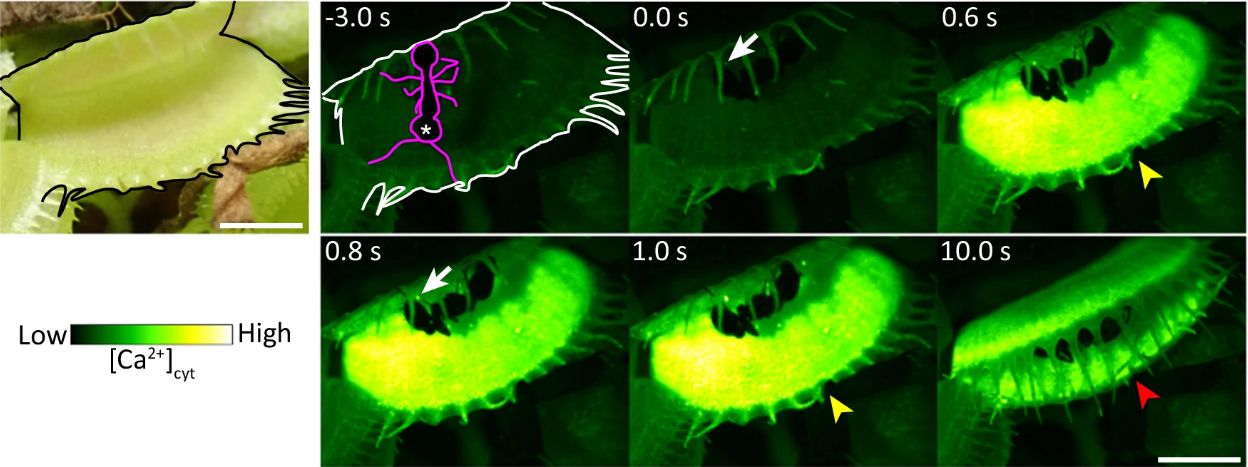
The Venus flytrap, a notable carnivorous plant, uses a spring-loaded trap mechanism to ensnare prey. Previous studies showed that its sensory hairs react to stimuli by triggering the release of calcium ions, but the specific mechanism remained unclear. This new research provides evidence that DmMSL10, a stretch-activated chloride ion channel, is fundamental to this process.
Understanding the Mechanism
In the study, the research team bred a variant of the Venus flytrap that lacked the DmMSL10 channel. They observed that while both the wild type and the knockout variant responded to mechanical stimulation, the generation rate of action potentials was significantly lower in the knockout variant. The wild type continued to produce action potentials even after mechanical stimulation ceased, highlighting the importance of DmMSL10 in processing even slight stimuli from the plant’s sensory hairs.
To further illustrate these findings, the team conducted experiments with ants. In these tests, the wild type Venus flytrap successfully captured the first ant that wandered onto its leaves. In contrast, the knockout variant failed to respond, with four successive ants not triggering enough calcium signal to close the trap. This stark difference underscores the critical role of DmMSL10 in prey detection.
Implications for Plant Biology
The discovery of DmMSL10 as a mechanosensor enhances our understanding of how plants like the Venus flytrap and other carnivorous species, such as the waterwheel plant (Aldrovanda vesiculosa) and sundews, have evolved to capture prey. It opens up new avenues for research on how these mechanisms may have evolved in parallel with similar processes in animals.
As scientists continue to explore the intricate relationships between plants and their environments, this finding could lead to broader insights into the evolutionary adaptations that allow carnivorous plants to thrive in nutrient-poor conditions. Understanding these mechanisms not only enriches our knowledge of plant biology but also deepens our appreciation for the complex interactions within ecosystems.