Researchers at Rice University have developed an innovative approach to manage seizure-related brain activity using a combination of sound waves and gene therapy. This new method offers a non-invasive alternative to traditional surgical procedures for treating epilepsy by targeting specific brain circuits with high precision.
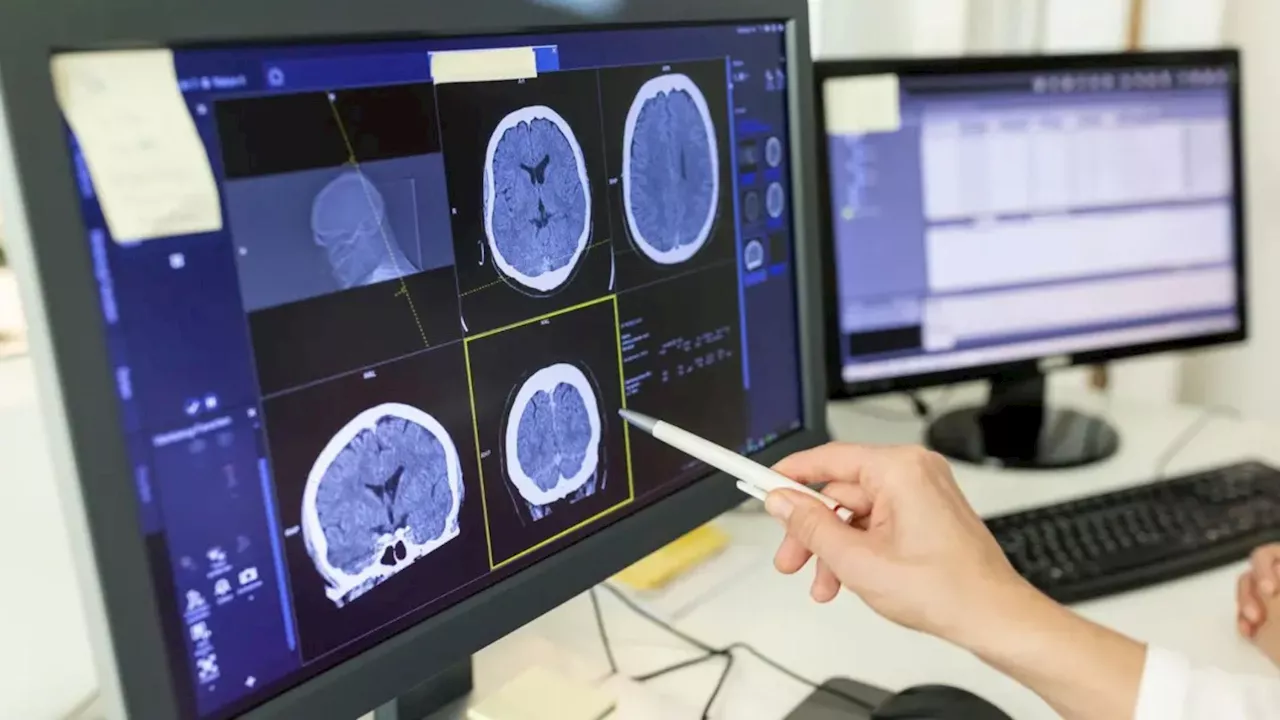
The study, led by Jerzy Szablowski, an assistant professor of bioengineering, demonstrates how low-intensity focused ultrasound can temporarily open the blood-brain barrier to deliver gene therapy directly to the hippocampus, a region frequently associated with seizure activity. This technique enables precise modulation of neuronal activity without affecting surrounding brain areas.
In their experimental setup, researchers used tiny, gas-filled bubbles injected into the bloodstream. When ultrasound waves were directed at the hippocampus, these bubbles created temporary openings in the blood-brain barrier, allowing gene therapy vectors to penetrate the targeted tissue. This process is crucial as it allows for the controlled delivery of genetic instructions that can either activate or deactivate specific neurons.
“This approach aims the therapy where it is needed and allows you to control it when you need it, without surgery and without a permanent implant,” Szablowski explained. The methodology, referred to as acoustically targeted chemogenetics (ATAC), combines ultrasound technology with gene therapy and chemogenetics, providing an effective means to tackle neurological disorders.
The gene therapy vectors carry instructions for an inhibitory chemogenetic receptor, functioning as a molecular “dimmer switch.” This allows researchers to fine-tune neuronal activity in the hippocampus, effectively dampening excessive brain activity while leaving other areas of the brain unaffected. Honghao Li, a doctoral student in bioengineering and the first author of the study, highlighted the significance of this targeted approach: “By precisely targeting the hippocampus, we can dampen overactivity where it matters and leave the rest of the brain untouched.”
The findings of this research, published in the journal ACS Chemical Neuroscience, indicate that the ATAC method can control specific brain circuits safely and with minimal invasiveness. This approach not only has the potential to transform epilepsy treatment but could also lead to advancements in therapies for other neurological disorders.
The researchers believe that since both focused ultrasound and viral vector gene delivery are currently being evaluated in clinical trials, their method could expedite the introduction of new treatments for patients suffering from epilepsy and similar conditions.
Szablowski’s team has made further strides in brain therapy by developing additional ultrasound-based techniques, such as recovery of markers through insonation (REMIS). This method allows for the release of proteins from targeted brain areas into the bloodstream for monitoring purposes.
“Ultrasound lets us deliver therapy, control the neurons we want, and then measure the effects in the exact circuit we targeted,” Szablowski noted. The long-term vision is to create a versatile platform that safely reaches any brain region, allowing for precise delivery of genetic material and on-demand control by clinicians.
This groundbreaking research underscores Rice University’s commitment to advancing brain science and neurological health, now consolidated under the newly established Rice Brain Institute. As the understanding of brain function continues to evolve, innovative techniques like ATAC pave the way for more effective and less invasive treatment options for patients worldwide.