Research led by Prof. Xia Yufei from the Institute of Process Engineering at the Chinese Academy of Sciences has introduced a novel approach to enhance immune responses in vaccine design. The study, published on January 8, 2026, in the journal Cell Biomaterials, demonstrates how deformable adjuvants can significantly improve the activation of immune cells, particularly in elderly and immunocompromised populations.
Conventional vaccine adjuvants often rely on molecular binding and biochemical stimulation to trigger immune responses. This method frequently yields limited effectiveness, especially among older adults or those with weaker immune systems. Recognizing the need for a new strategy, the research team focused on integrating physical regulation into the immune activation process.
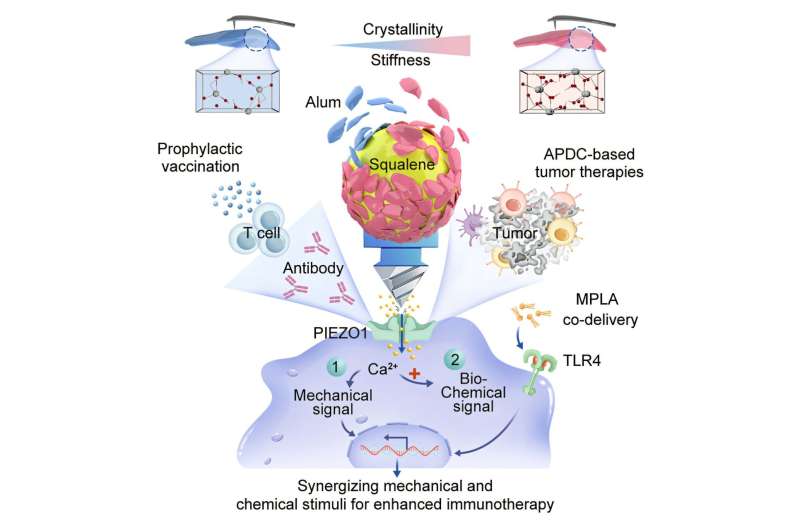
The researchers engineered aluminum-stabilized Pickering emulsions (ASPEs) that allow dendritic cells (DCs) to interact with these droplets in a dynamic manner. Unlike traditional particulate adjuvants, which have restricted contact with DC membranes, ASPE droplets deform upon membrane contact. This deformation increases the interfacial contact area and applies controllable mechanical stress, enhancing the immune response.
The team discovered that by adjusting the crystallinity of aluminum nanoparticles, they could finely regulate the interfacial stiffness of these emulsions. This innovation enables graded mechanical stimulation of the immune system. Stronger mechanical cues activate the mechanosensitive ion channel PIEZO1, triggering an influx of calcium ions (Ca2+) and promoting effective antigen cross-presentation.
When combined with the TLR4 agonist monophosphoryl lipid A (MPLA), the ASPE platform provides synergistic mechano-biochemical activation. This combination outperformed the standard clinical formulation of Alum+MPLA, leading to stronger maturation of dendritic cells and enhanced Th1-biased immunity, alongside robust responses from CD8+ T-cells.
The research findings are particularly significant in the context of aging populations. In experiments involving aged mouse models, the ASPE-M formulation improved therapeutic outcomes in dendritic cell-based melanoma immunotherapy, especially when paired with PD-1 blockade therapies.
Overall, this study establishes the importance of interfacial mechanics as a programmable dimension of immune regulation, which complements traditional biochemical signaling pathways. By redefining how adjuvants function, the research offers promising new strategies for vaccine and immunotherapy design, particularly aimed at improving immune efficacy in vulnerable populations.
The implications of this work extend far beyond basic science, potentially transforming how vaccines are developed and administered to those most in need of effective protection.
Further details can be found in the study by Yali Ming et al., titled “Drilling dendritic cell activation: Engineering interfacial mechano-biochemical cues for enhanced immunotherapy,” published in Cell Biomaterials.