A study from Baylor College of Medicine has revealed that certain mutations in the p53 gene may enhance the effectiveness of immunotherapy in cancer treatment. This groundbreaking research focuses on two specific p53 mutations, R273H and R175H, and how they interact with cancer cell behavior and immune responses. The findings, published in Communications Biology, suggest that understanding these mutations could significantly influence treatment strategies for cancer patients.
The p53 gene is known as the “guardian of the genome” due to its role in maintaining genomic stability. When mutated, however, it can promote tumor growth rather than suppress it. Approximately 50% of all human cancers involve some form of p53 mutation. Despite its central role in cancer biology, the implications of specific p53 mutations for treatment have remained largely unexplored.
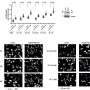
In the study, the research team, led by Dr. Weei-Chin Lin, professor of molecular and cellular biology, investigated how the R273H and R175H mutations affected cancer cell growth in laboratory settings. Dr. Lin noted, “We took a closer look at how each of these mutants affected different steps of the complex mechanism of DNA replication.” The team found that the R273H mutation led to excessive DNA replication, which in turn spurred aggressive cancer cell proliferation. Surprisingly, this excessive replication also triggered a strong immune response against the cancer cells by activating the cGAS-STING pathway, a crucial component of the body’s innate immune defense.
In contrast, the R175H mutation did not elicit an immune response, despite its promotion of cancer growth. This stark difference emphasizes the need for clinicians to understand the specific type of p53 mutation present in a patient’s tumor, as it may significantly impact both tumor behavior and potential treatment options.
To explore the potential for immunotherapy, the researchers conducted tests on mouse models of breast cancer. They evaluated whether tumors with the R273H mutation responded better to immune checkpoint inhibitors, a type of therapy designed to enhance the immune system’s ability to combat cancer. The results showed that tumors harboring the R273H mutation responded positively to this treatment, exhibiting an increase in CD8+ T cells—immune cells that play a pivotal role in targeting cancer.
Dr. Lin stated, “Although more studies are needed before these findings can be implemented in the clinic, they offer the possibility that doctors might be able to predict which patients will respond better to immunotherapy by identifying tumors with mutant p53 variants like R273H.” The research indicates that combining immunotherapy with drugs that specifically target DNA replication could further amplify the immune response in patients with specific p53 mutations.
As cancer treatment continues to evolve, this study opens new avenues for personalized therapy tailored to the genetic profiles of tumors. The work of Kang Liu, Lidija A. Wilhelms Garan, and Fang-Tsyr Lin, all contributors from Baylor College of Medicine, represents a significant step forward in understanding how p53 mutations can be strategically exploited in the fight against cancer.
Future research aims to develop these findings into practical applications that may enhance treatment efficacy and improve outcomes for patients diagnosed with cancer.