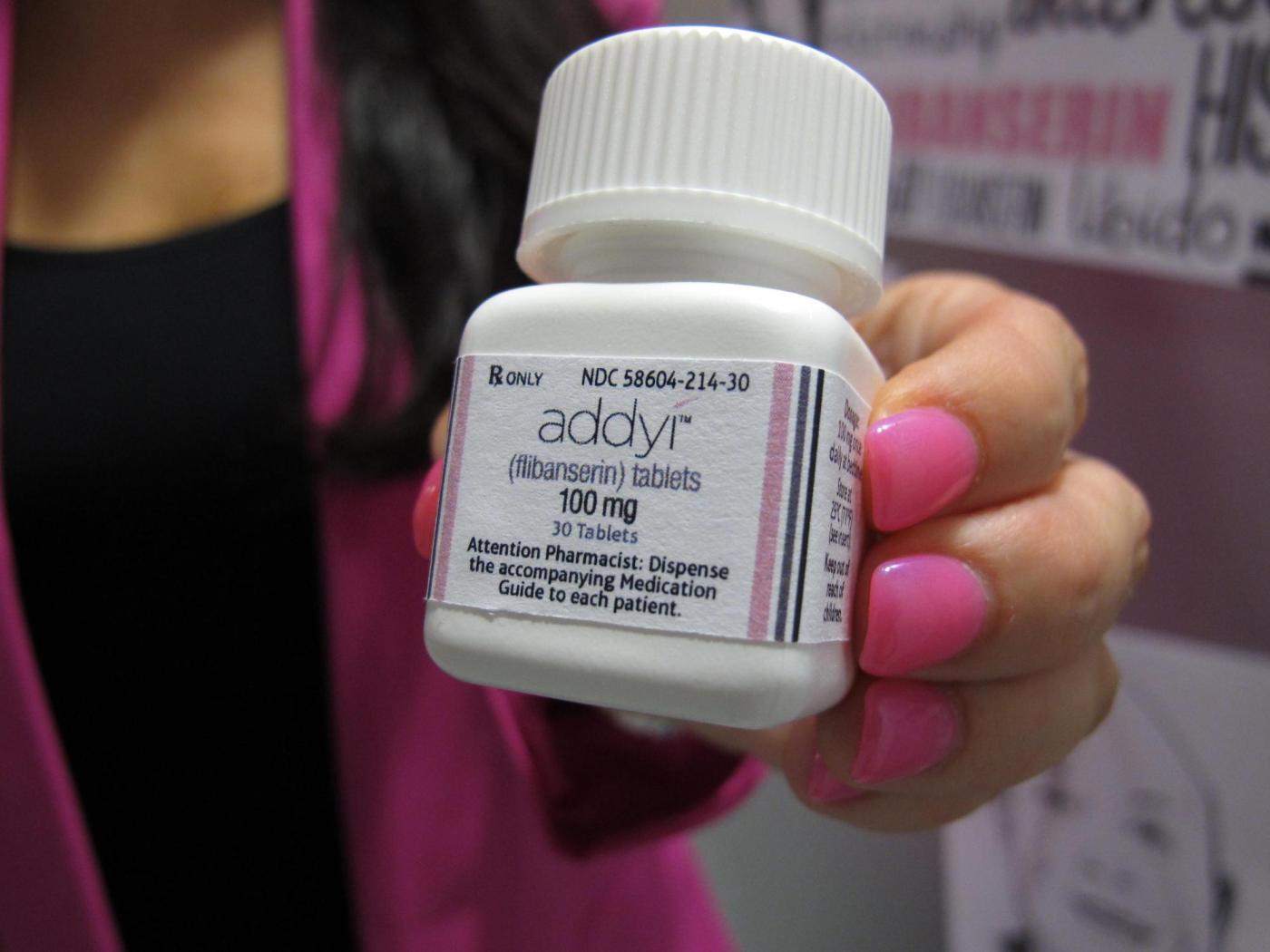
U.S. health officials have announced an expansion of the approval for Addyi, a medication aimed at increasing libido in women who have gone through menopause. The U.S. Food and Drug Administration (FDA) stated on March 4, 2024, that the once-a-day pill can now be prescribed to women over the age of 65, a significant shift in its intended demographic.
Initially approved in 2015 for premenopausal women experiencing distress due to low sexual desire, Addyi has faced considerable scrutiny over the years. The drug, marketed by Sprout Pharmaceuticals, has been met with mixed reactions due to its side effects, which include dizziness and nausea. Moreover, it carries a serious safety warning against alcohol consumption, as combining the two can lead to dangerously low blood pressure and fainting.
Despite the initial expectations that Addyi would become a significant player in women’s health, its sales have been limited. This is partly attributed to the complex nature of diagnosing hypoactive sexual desire disorder, a condition recognized since the 1990s. Surveys suggest that a notable portion of American women may experience this condition, yet diagnosing it is fraught with challenges. Factors influencing libido can vary widely, particularly after menopause, where hormonal changes can trigger various biological and psychological symptoms.
In a statement following the FDA’s announcement, Cindy Eckert, CEO of Sprout Pharmaceuticals, expressed her satisfaction with the decision. She noted that the approval reflects a decade of persistent efforts to reshape the understanding and prioritization of women’s sexual health. This expansion of approval is seen as a recognition of the need for more comprehensive options for women facing sexual health issues.
The FDA’s approval of Addyi was not without its challenges. The agency previously rejected the drug twice due to concerns over its modest effectiveness and potential side effects. The eventual approval came after a lobbying campaign led by Sprout and its supporters, who framed the lack of treatment options for female libido as a significant women’s rights issue.
In 2019, the FDA also approved a second drug for low female libido, an injectable medication that acts on a different set of neurological chemicals. This added another layer of options for women seeking remedies for sexual dysfunction.
While the medical community recognizes hypoactive sexual desire disorder, the diagnosis remains contentious. Many psychologists argue against classifying low libido as a medical problem, suggesting that relationship dynamics, mental health issues, and other factors should be thoroughly evaluated before medication is considered.
As the conversation surrounding women’s sexual health continues to evolve, the FDA’s recent decision marks a notable development. It highlights the ongoing need for research and discussion around female sexual dysfunction, an area that has historically been under-prioritized compared to male counterparts. The expansion of Addyi’s approval could offer hope and improved quality of life for many women navigating the complexities of menopause and sexual health.