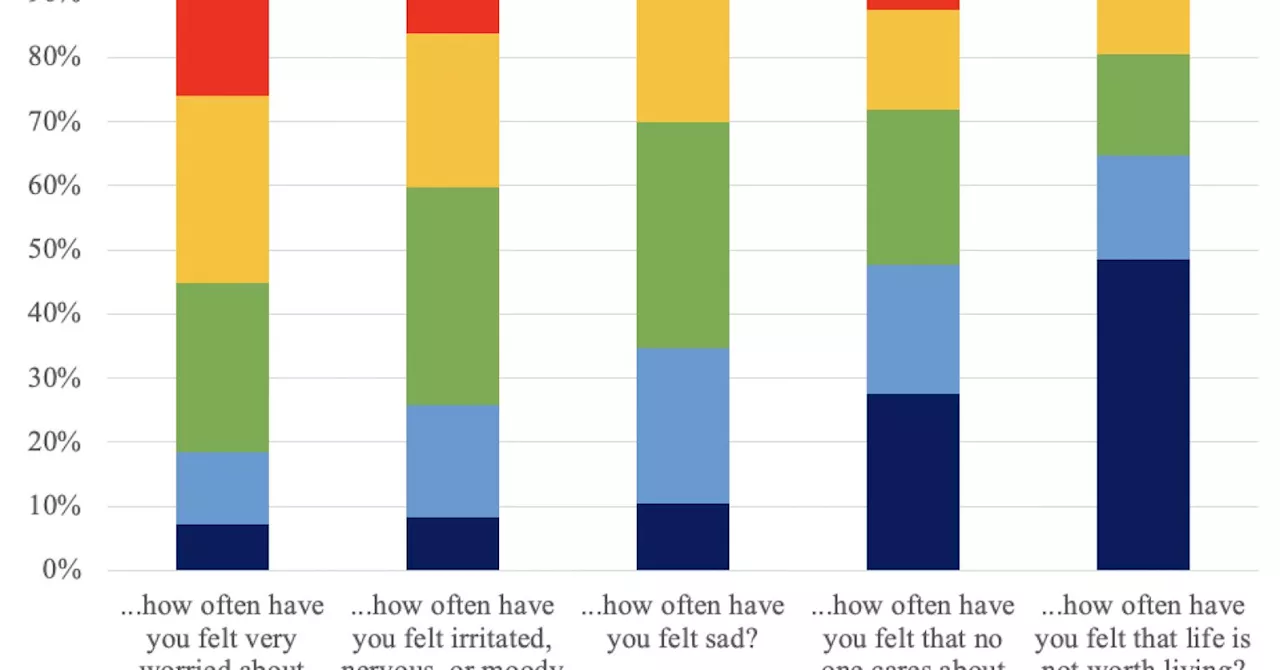
Biohaven Pharmaceuticals announced that its potassium channel drug has failed a Phase 2 trial aimed at treating patients with major depressive disorder (MDD). The trial, which concluded in September 2023, did not meet the primary endpoints set by the company, raising concerns about the drug’s efficacy in addressing this serious mental health condition.
The drug, initially designed to target ion channels, was part of Biohaven’s broader effort to develop innovative treatments for various neurological and psychiatric disorders. This setback marks the second failure for Biohaven in the MDD space, following an earlier disappointment in a different trial that resulted in a similar outcome.
Implications for Biohaven and the Market
The failure of this drug could have significant implications for Biohaven’s future, particularly as the company seeks to establish itself within the competitive pharmaceutical landscape. Investors have expressed concerns, reflected in a decline in the company’s stock price following the announcement. Biohaven has yet to provide a detailed analysis of the trial results, leaving many in the industry speculating about the next steps for the drug and the company.
According to Biohaven, the Phase 2 trial was designed to assess both the safety and efficacy of the medication in a controlled environment. The company recruited a diverse population of participants to ensure the results would be applicable across various demographics. Despite these efforts, the results did not support the initial hypothesis that the drug could significantly improve symptoms associated with major depressive disorder.
Future Directions for Biohaven
In light of this recent failure, Biohaven is now faced with critical decisions regarding its research and development pipeline. The pharmaceutical industry is known for its high risk and high reward nature, where many compounds fail to translate from preclinical studies to successful treatments. Biohaven’s management may need to reevaluate its focus on ion channel modulation and consider alternative strategies or therapeutic areas.
Investors and stakeholders will be closely monitoring Biohaven’s next moves. The company has previously shown resilience in the face of setbacks, leveraging its existing portfolio and pipeline to pivot towards new opportunities. However, the road ahead may be challenging as Biohaven works to regain investor confidence and explore new therapeutic avenues.
As the pharmaceutical landscape continues to evolve, the outcomes of Biohaven’s trials serve as a reminder of the complexities involved in drug development. The firm’s ability to learn from these experiences will be crucial in determining its future success in the industry.