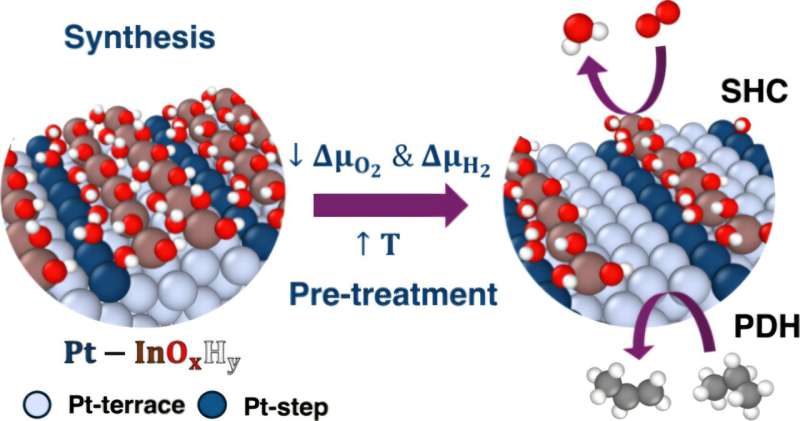
A team of researchers at the University of Rochester has developed innovative algorithms that clarify the complex chemical processes involved in converting propane into propylene. This breakthrough, detailed in a study published in the Journal of the American Chemical Society on November 13, 2025, could significantly enhance the efficiency of industrial production methods used for a variety of everyday products, including plastics and fuels.
Revolutionizing Chemical Production
Propylene is a key component in numerous consumer goods, ranging from plastic squeeze bottles to outdoor furniture. Traditionally, the transformation of propane to propylene involved intricate methods that were often inefficient. A study published in 2021 highlighted the use of tandem nanoscale catalysts to streamline this process. Yet, the atomic-level details remained elusive, complicating the broader application of these techniques across different industrial reactions.
In response to this challenge, researchers led by Siddharth Deshpande, an assistant professor in the Department of Chemical and Sustainability Engineering, created algorithms that identify the essential atomic features that govern the chemistry of these nanoscale catalysts. Deshpande emphasized the necessity of this algorithmic approach, stating, “There are so many different possibilities of what’s happening at the catalytic active sites, so we need an algorithmic approach to very easily yet logically screen through the large amount of possibilities that exist and focus on the most important ones.”
Key Findings and Implications
Deshpande, along with Ph.D. student Snehitha Srirangam, conducted an extensive analysis of the metallic and oxide phases involved in the reaction. Their findings uncovered surprising behaviors, including the tendency of oxide to preferentially accumulate around defective metal sites, which is crucial for maintaining catalyst stability. Remarkably, although the oxide can vary in its chemical composition, it consistently performs its function near these defective sites.
“Our approach is very general and can open the doors to understanding many of these processes that have remained an enigma for decades,” Deshpande remarked. “We know these processes work, and we produce tons of these chemicals, but we have much to learn about why exactly they’re working.”
This research not only sheds light on the propane to propylene conversion but also holds promise for advancing understanding of other chemical reactions, such as methanol synthesis, which is vital for producing products ranging from paints to fuel cells. The algorithms developed by the Rochester team could help industries move away from traditional trial-and-error methods, potentially leading to more efficient pathways for the production of essential materials.
Deshpande believes that by leveraging these insights, companies can strategically enhance their production techniques, ultimately benefiting both the economy and the environment. The study represents a significant step forward in chemical engineering, providing a clearer understanding of complex processes that underpin many industrial applications.
For further details, refer to the publication: Snehitha Srirangam et al, Site-Selective Oxide Rearrangement in a Tandem Metal–Metal Oxide Catalyst Improves Selectivity in Oxidative Dehydrogenation of Propane, Journal of the American Chemical Society (2025). DOI: 10.1021/jacs.5c13571.