Scientists at Stanford University have achieved a remarkable breakthrough by pushing iron into a higher-energy state than previously possible, potentially revolutionizing battery technology. This advancement may lead to the development of more efficient and cost-effective batteries, which could significantly impact the future of energy storage solutions.
The research team, led by PhD students Hari Ramachandran, Edward Mu, and Eder Lomeli, collaborated with 23 other researchers from various U.S. universities, national laboratories, and international partners in Japan and South Korea. Their findings indicate that iron can release and reabsorb more electrons than earlier thought, suggesting a new path for batteries that are not only more powerful but also cheaper than current cobalt- or nickel-based models.
Innovative Techniques in Material Science
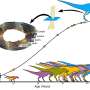
The team discovered a method to manipulate the structure of a compound made from lithium, iron, antimony, and oxygen. When designed at the nanoscale, this material allowed iron atoms to reversibly lose and regain five electrons, surpassing the typical two or three electrons expected from iron.
Initially, Ramachandran and Mu faced challenges as their early samples collapsed during charging cycles. They deduced that reducing the particle size to between 300 and 400 nanometers—approximately 40 times smaller than previous attempts—was essential. This breakthrough came from their ability to grow crystals from a precisely mixed liquid solution.
“In our electrochemical tests, the material seemed to get iron to reversibly give up and later take back five electrons while the crystal structure remained stable,” Mu explained, highlighting the stability of their innovation.
To confirm the workings of this novel state, Lomeli collaborated with Tom Devereaux, an expert in modeling X-ray spectra. Their analysis revealed that the additional electrons were not solely derived from iron atoms; rather, oxygen played a crucial role. “It’s too simple to say that iron is the hero or oxygen is the hero,” Lomeli noted. “The atoms in this well-arranged material behave like a single entity.”
A New Era for Iron in Battery Technology
The emergence of iron as a viable option in battery technology marks a significant shift. Previously considered unsuitable for advanced energy storage due to its low-voltage characteristics, iron-based cathodes are now being viewed as sustainable alternatives to cobalt, which is both costly and often mined under unsafe conditions. “A high-voltage, iron-based cathode could avoid the tradeoff between higher voltage and higher-cost metals that previously dominated cathode materials,” Mu stated.
This concept of enhancing iron’s capabilities traces back to research by former Stanford PhD student William Gent, who theorized in 2018 that the spacing of neighboring atoms could allow iron to reach higher oxidation states. Although Gent did not have the opportunity to complete his experiment, the current team has successfully brought this theory to fruition. Early tests conducted at the SLAC-Stanford Battery Center demonstrated that the lithium-iron-antimony-oxygen compound maintained structural integrity while bending slightly during charge cycles.
Co-lead author and researcher William Chueh remarked, “Scientists have rarely reported high-voltage iron-based materials. Our detailed electronic structure exploration of this iron species provides conclusive evidence of oxidation beyond three electrons.” The full study detailing these findings was published in Nature Materials earlier this month.
This advancement in iron’s application in energy storage not only holds promise for low-cost batteries but also has implications for various technologies that depend on magnetic and electronic properties, such as MRI machines and maglev trains. As the demand for sustainable energy solutions continues to rise, this research paves the way for more accessible and environmentally friendly battery technologies.