Researchers at Beihang University have developed a new biochar material that efficiently removes stable metal complexes from water. This innovative solution, known as ferromanganese oxide-modified biochar (FMBC-600), exhibits an impressive copper removal rate of 99.5% and a total organic carbon reduction of 92.6%. The study, published in Biochar X on October 14, 2025, addresses a critical challenge in wastewater treatment, particularly concerning persistent metal–organic pollutants.
Conventional treatment methods often focus on free metal ions, neglecting the stable metal complexes that dominate industrial and municipal effluents. These complexes, frequently bound with organic agents like citric acid, resist degradation and can pose long-term ecological and health risks. Industries such as electroplating and textile dyeing commonly produce copper-citrate complexes, which are difficult to remove using traditional precipitation and ion exchange techniques.
The FMBC-600 was synthesized through a process of impregnation and high-temperature calcination, allowing researchers to enhance its adsorption capability and stability. The team, led by Wenhong Fan, thoroughly characterized the modified biochar, revealing a rough surface coated with nanoparticles measuring 80–100 nanometers. The incorporation of iron and manganese was confirmed through energy-dispersive spectroscopy (EDS), while Fourier-transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD) analyses identified various functional groups and crystalline phases essential for adsorption.
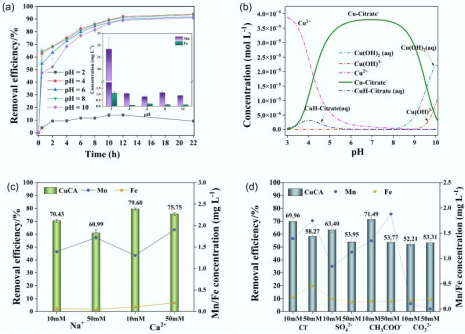
The study highlights the adsorption mechanism, which combines chemical bonding via oxygen-containing functional groups with physical adsorption facilitated by the biochar’s porous structure. The nitrogen adsorption-desorption analysis indicated an increased pore volume, further enhancing its performance. Optimal conditions for copper removal were identified as an iron to manganese ratio of 1:4, manganese concentration of 0.03 M, and pyrolysis at 600 °C.
Under these conditions, the FMBC-600 achieved rapid adsorption, with copper removal occurring within 30 minutes. It maintained stability across a range of pH levels from 4 to 10, demonstrating resilience even in the presence of competing ions such as sodium, calcium, and sulfate. Kinetic modeling indicated that chemisorption was the dominant process, while isotherm studies showed heterogeneous multilayer adsorption that improved at higher temperatures.
The regenerative capacity of the FMBC-600 was also notable, maintaining about 80% efficiency after two cycles of use. This remarkable stability and reusability position the material as a scalable and cost-effective option for addressing heavy metal contamination in wastewater.
The implications of this research extend beyond wastewater treatment. The ferromanganese oxide-modified biochar can be adapted for soil remediation, potentially reducing heavy metal accumulation in agricultural lands. Its production process is simple and low-cost, making it feasible for large-scale applications in various industries.
By offering superior selectivity and performance under varying conditions, the FMBC-600 represents a significant advancement in sustainable water treatment technologies. The research is backed by the National Natural Science Foundation of China, among other funding sources, ensuring its credibility and potential impact on global clean water initiatives. This breakthrough aligns with the urgent need to improve wastewater management practices and achieve sustainability goals worldwide.